WebDCU™ Overview
DCU has broad experience with designing and implementing biomedical/clinical information systems for single and
multi center research studies. DCU has successfully developed more than 40 clinical trial information systems, which are equipped with cutting edge and secure computer technology.
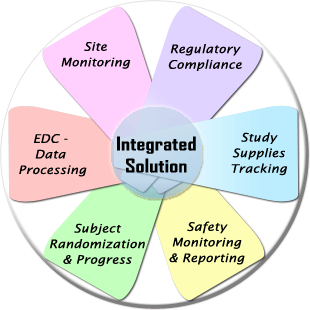 WebDCU™ system is an integrated web-based central information system developed by the Data Coordination Unit (DCU) for clinical trial data management as well as full scope trial operation management.
In addition to the Electronic Data Capture (EDC) function, it provides numerous clinical trial project operation management tools, including subject randomization, drug tracking, medical safety reporting, data monitoring, regulatory document management, and subject payment management.
WebDCU™ system functionalities include
WebDCU™ system is an integrated web-based central information system developed by the Data Coordination Unit (DCU) for clinical trial data management as well as full scope trial operation management.
In addition to the Electronic Data Capture (EDC) function, it provides numerous clinical trial project operation management tools, including subject randomization, drug tracking, medical safety reporting, data monitoring, regulatory document management, and subject payment management.
WebDCU™ system functionalities include

- Electronic Data Capture (EDC): Case Report Form Data Processing Module
- Study Design: Defining study visits, visit transition logic, data collection schedule etc.
- Subject Randomization and Progress Monitoring Module
- Safety Monitoring and Reporting Module
- Study Material Tracking Module
- Regulatory and Project Document Management Module
- Financial Management Module
- Site Monitoring Module
- Network Management: Managing projects, institutions, users, user roles and permissions
- Community Engagement Activities: Tracking community consultation and public disclosre activities
- Real-time Reports: Customized, complex logic reports


