WebDCU™: Subject Randomization and Progress Module
Centralized Subject Randomization
WebDCU™ provides a secure central randomization support with 24/7 accessibility through the internet. A central randomization strategy enhances the treatment allocation blinding protection and reduces the likelihood of selection bias associated with local randomization, while allows advanced randomization algorithms to be implemented including Minimal Sufficient Balancing and response-adaptive randomization. With the integration of the randomization algorithm within the WebDCU™ system, subject randomization information can be seamlessly shared within the study community in real time to optimize the trial operation management.
Before performing a subject randomization, WebDCU™ checks the subject’s eligibility and baseline covariate information, if applicable. While these data are captured on relevant CRFs, no redundant data entry is needed for subject randomization. Automated cross checking of the CRFs ensures that ineligible patients are blocked from randomization. When a new subject is randomized, an automated email is sent to relevant study team members allowing real-time monitoring of the study recruitment progress.
DCU provides 24-hour hotline support for questions associated with randomization.
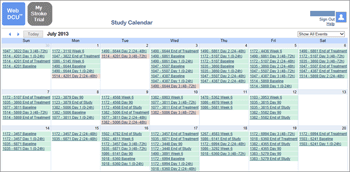
Subject Progress Monitoring
During the CRF development stage, protocols are broken down into distinct visits, such as baseline, treatment, follow-up and end-of-study. When a new subject is enrolled in the study, a study calendar is generated by the system with the projected target date for each subsequent study visit. A monthly study calendar is composed by the WebDCU™ with color coded items for completed, pending and overdue subject visits, allowing sites to efficiently manage subject visit scheduling, treatment and assessment.