WebDCU™: Electronic Data Capture and Management Module
Case Report Form (CRF) processing is the core of clinical trial data management. The below CRF processing flow chart shows our CRF processing procedure for a typical clinical trial.
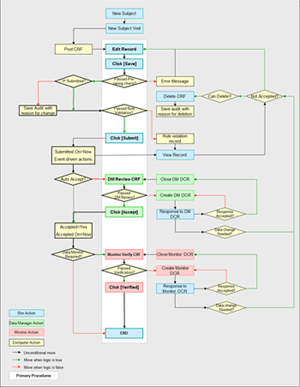 All CRF data are entered directly by authorized study team members at participating clinical sites through WebDCU™ user interfaces, which have data type format controls, skip patterns, and conditional selections programmed based on basic data logic. Initial data checking is performed by the system before the data can be saved to prevent low level data errors, such as entering text into a numeric data field.
After the data is saved, rule-based data validation, including data range checks, data consistency checks and within/cross form logic checks, is conducted based on common logic and the study protocol. Potential data errors and protocol violations are flagged on the user interface, requesting the data entry person to confirm or correct the data based upon the source document. Only CRFs without unresolved rule violations are eligible for submission.
Although this requires intense programming in the initial stages of the study, the overall efficiency of being able to resolve data queries at the data entry phase and at the site level with a full audit trail proves
to be a valuable system.
Once submitted, CRFs are reviewed by DCU data managers. When needed, Data Clarification Requests (DCRs) are created by the data manager. Sites are expected to respond to each DCR and edit the CRF data, as needed. DCR responses are reviewed by the data manager, who can either close the DCR or request further clarification. All DCR processing activities are coordinated in WebDCU™ with event driven email notifications. All CRF data edits including the reason for the data change and time stamps, are archived in the audit trial.
Dependent on the requirements of the study, certain data may be monitored by an on-site data monitor against source documents. Should a data discrepancy between the source document and database be identified, a DCR will be generated by the data monitor.
When CRF data are transferred to the study biostatisticians for analysis and report generation, further data quality checks are conducted by biostatisticians in SAS® or other statistical software. Findings regarding CRF data quality are transferred to the data management team and resolved though the DCR channel.
One caveat to web-based data management is its dependence on the timeliness of data entry at the clinical sites. Site specific CRF processing summary reports and detailed missing or late CRF lists are also available in WebDCU™, allowing DCU data managers to carefully monitor the study data collection activities across all sites and to ensure that data collection is preceding uniformly and efficiently.
All CRF data are entered directly by authorized study team members at participating clinical sites through WebDCU™ user interfaces, which have data type format controls, skip patterns, and conditional selections programmed based on basic data logic. Initial data checking is performed by the system before the data can be saved to prevent low level data errors, such as entering text into a numeric data field.
After the data is saved, rule-based data validation, including data range checks, data consistency checks and within/cross form logic checks, is conducted based on common logic and the study protocol. Potential data errors and protocol violations are flagged on the user interface, requesting the data entry person to confirm or correct the data based upon the source document. Only CRFs without unresolved rule violations are eligible for submission.
Although this requires intense programming in the initial stages of the study, the overall efficiency of being able to resolve data queries at the data entry phase and at the site level with a full audit trail proves
to be a valuable system.
Once submitted, CRFs are reviewed by DCU data managers. When needed, Data Clarification Requests (DCRs) are created by the data manager. Sites are expected to respond to each DCR and edit the CRF data, as needed. DCR responses are reviewed by the data manager, who can either close the DCR or request further clarification. All DCR processing activities are coordinated in WebDCU™ with event driven email notifications. All CRF data edits including the reason for the data change and time stamps, are archived in the audit trial.
Dependent on the requirements of the study, certain data may be monitored by an on-site data monitor against source documents. Should a data discrepancy between the source document and database be identified, a DCR will be generated by the data monitor.
When CRF data are transferred to the study biostatisticians for analysis and report generation, further data quality checks are conducted by biostatisticians in SAS® or other statistical software. Findings regarding CRF data quality are transferred to the data management team and resolved though the DCR channel.
One caveat to web-based data management is its dependence on the timeliness of data entry at the clinical sites. Site specific CRF processing summary reports and detailed missing or late CRF lists are also available in WebDCU™, allowing DCU data managers to carefully monitor the study data collection activities across all sites and to ensure that data collection is preceding uniformly and efficiently.



